Elmiron eye damage and vision loss lawsuits are now in an MDL class action lawsuit that houses all federal court Elmiron lawsuits. Our lawyers are seeking new victims who are seeing a settlement compensation payout for their injuries in all 50 states.
Our Elmiron lawyers believe these class action eye damage lawsuits will ultimately result in large settlements and, just as important, a new and strong warning that lets doctors and patients know of the risks of Elmiron. Our Elmiron lawyers are handling these cases throughout the country.
There is an Elmiron lawsuit that will go to trial in the class action in 2023. There could be a class action settlement before that trial… or possibly a large verdict that will further ignite this litigation.
Our law firm is reviewing Elmiron vision loss lawsuits. This is not a huge class action. But as of December 2022, there are over 1,800 filed Elmiron lawsuits in the class action. This is national litigation and our law firm is reviewing these cases in every state in the United States. If you have a claim, call our lawyers at 800-553-8082. You can also reach out to me online. There is no cost or fee unless our law firm gets a recovery for you.
What is the Elmiron lawsuit about?
Elmiron, the brand name for the drug pentosan polysulfate sodium (PPS), is used to treat interstitial cystitis (IC), a chronic and incurable bladder condition. IC, which primarily affects women, causes mild to severe pain and pressure in the bladder area.
Having IC is like having a bladder infection that never goes away, and the condition can also cause lower urinary tract symptoms and pain during sex. IC is also known as bladder pain syndrome (BPS). Currently, Elmiron is the only drug approved by the FDA specifically to treat IC, though there are other medications and treatments.
According to Janssen Pharmaceuticals (Johnson & Johnson), the drug’s manufacturer, IC is believed to be caused by damage to the bladder’s lining. Painful symptoms arise because substances in the urine irritate the damaged spots. Pentosan polysulfate sodium, the Elmiron drug, is a blood thinner.
PPS seems to attach to the bladder wall, protecting the damaged areas of the bladder wall from outside irritants. In this way, Elmiron is sort of like a band-aid, in how covering a cut with a band-aid immediately relieves pain and prevents it from further irritation.
Elmiron lawsuits come from the knowledge we now have that links Elmiron to maculopathy, a disease of the macula region of the eye. The macula is responsible for sharp, color vision in the center of the field of vision. Maculopathy is one of the most common causes of blindness. Researchers are calling the vision problems that result from taking Elmiron pentosan polysulfate maculopathy (PPM).
What’s new in the Elmiron lawsuits in December 2022?
The biggest new thing is that we now have a date for the first Elmiron trial. You won’t be happy. The first trial will not be until the spring of 2023 after getting pushed back from January 2023. Things move slowly in class action lawsuits and I’m sorry about this.
But having a trial date does begin to hold the defendants’ feet to the fire. It has always been my view that an Elmiron lawsuit will never go to trial before there is a global class action settlement that resolves most of the Elmiron lawsuits.
The conventional wisdom is that these lawsuits will settle in 2023 and the average Elmiorn lawsuit will fetch beween $300,000 and $500,000. Will this come to pass? We will see?
What are the Elmiron lawsuits about?
About 1 million people suffer from IC in the United States. Elmiron was approved by the FDA in 1996, but its effect on vision was only uncovered in 2018. If you’re wondering how this happened, you’re not alone. Didn’t the clinical trials reveal that Elmiron damaged the eyes? Why weren’t patients and doctors warned about this potential effect?
So far, the answers to these questions do not look reassuring. You, as a victim, could be owed compensation for the irresponsibility of these pharmaceutical companies. On March 2020, the first lawsuit was filed over Elmiron eye damage. The plaintiff allegedly suffered vision loss.
Filed in Connecticut, the defendant is Teva Branded Pharmaceuticals R&D. The suit alleges that the makers of Elmiron (1) did not warn patients or the medical community about the danger, (2) withheld information from clinical trials about the danger, and (3) failed to take the drug off the market once they were aware of the danger. The drug is still on the market as you read this blog post.
Perhaps the most startling accusation, which we often see in these types of cases, is that there was evidence in one or more of the clinical trials performed before the drug was ever released that showed the possibility of eye damage occurring. It would be upsetting (but not surprising) to discover that these companies would overlook or even attempt to conceal information in order to sell their product.
The next step in these lawsuits was the gathering of Elmiron lawsuits from across the country and the consolidation of the cases into a class action. This has been done and all federal cases are now pending before one judge in New Jersey.
Did the makers of Elmiron know about this all along?
How did it take researchers so long to discover the connection between this drug and vision loss? I’m nonplussed. One of the reasons why this condition may have been discovered so late is that it requires chronic exposure to the drug.
It is possible that it took this long for people to acquire the condition. The condition, too, has somewhat uncertain symptoms and is easily misdiagnosed. Still, 22 years is a long time, right?
But perhaps more importantly, from a legal perspective, did the makers of Elmiron know about the connection and market their drug anyway? This is certainly going to be revealed and will be a crucial factor as lawsuits continue to be filed and pigmentary maculopathy lawyers dig into the case. Our lawyers have our suspicions. But litigation is going to give the final answer.
Another really important point is that the drug is still on the market. Janssen Pharmaceuticals should have addressed the issue at this point.
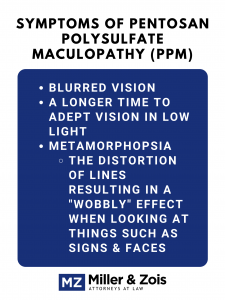
What are the symptoms of pentosan polysulfate maculopathy?
If you currently take, or at some point in your life took, Elmiron, you are at risk for vision problems. The most pressing question on your mind is, will I lose my vision? The good news is, according to a recent study, visual acuity is not necessarily affected in patients with PPM. This means that Elmiron is not causing all-out blindness.
However, patients in the study most commonly reported blurred vision, needing a longer time to adapt their vision to low light levels, and metamorphopsia. What is metamorphopsia? From the Greek for transformation, metamorphopsia is the distortion of lines that results in a wobbly effect when looking at things like signs and faces.
One study estimated that 24% of people who took Elmiron would experience eye damage; however, this number could be much higher. Clearly, even though PPM does not cause vision loss, it is a difficult and disruptive condition, especially for those who already suffer from chronic bladder pain.
What research has been done to link Elmiron to eye damage?
In 2018, doctors at the Emory Eye Center in Atlanta, Georgia discovered that six patients who were taking Elmiron also had maculopathy. The same damage was observable in most of their eyes and most had trouble with things like reading and seeing in the dark.
Could it just have been a coincidence that all these patients with identical eye damage were taking the same medication? It would be a striking coincidence, but to rule out that possibility, the doctors tested the patients to see if their eye damage was caused by something else. They found that none of the six people had a genetic mutation or infection-related variation in their DNA that would explain what the doctors were seeing.
The doctors’ suspicions were confirmed, and they published their results with the American Academy of Ophthalmology in November 2018.
However, researchers never make generalizations based on studies with a small number of participants. Larger studies were needed to determine whether Elmiron really caused maculopathy. In October 2019, researchers with Kaiser Permanente in Oakland, California released their much larger study of 91 patients, 22 of whom had signs of drug toxicity. Their findings suggested that the higher the dose of the drug you take, the higher the chances that your eyes will be damaged.
Many more studies are listed in the bibliography at the bottom of this page.
Why is this the first I’m hearing of the connection between these eye injuries and Elmiron?
Optometrist and ophthalmologists either do not know of the connection or they downplay the connection between eye damage and Elmiron. As a result, unless you stumble a TV commercial from an Elmiron lawyer, many people are simply unaware of the conversation in the medical literature about the potential connection.
December 2022 Elmiron Update
The was a trial set for next month. That has been postponed until March 27, 2022. My guess is that this case will settle before trial and that will trigger a settlement of most Elmiorn lawsuits. But that is just my speculation.
But the Elmiron lawsuits are thought to be strong. Every plantiffs’ lawyer I have spoken to is high on these cases. As discovery continues in the Elmiron litigation, mass tort lawyers usually see the medical literature turn one way or the other after lawsuits are filed. Plaintiff’s attorneys sometimes see what appears to be a great lawsuit fall apart as the science unfolds. I’ve seen lawsuits I was extremely high on only to learn that the developing science just did not support the claims. But in these cases, it looks like it is heading the plaintiffs’ way.
In a Clinical Ophthalmology-published study, Northwestern University researchers investigated the pigmentary maculopathy presence in Elmiron patients. They searched the university ophthalmology clinic’s electronic health records. The researchers narrowed their search to Elmiron patients who visited the clinic between 2002 and 2019.
The study identified 131 patients. Forty of them had imaging, while the remaining 91 had fundus examinations. The researchers found that five of the 40 with imaging and five of the 91 with fundus examinations showed signs of Elmiron-induced pigmentary maculopathy. The 10 patients with these signs took the drug for an average of 4.2 years at a cumulative dose average of 380 grams.
Based on their data, the researchers concluded that imaging can identify Elmiron-induced pigmentary maculopathy. This helps plaintiffs in the Elmiron lawsuits because it helps prove the injury was caused by Elmiron.
Plaintiffs’ experts in this litigation should have plenty of ammunition to fend off a Daubert challenge and get these cases to trial.
Example Elmiron Lawsuit
To put this in some context, let’s look at a recently filed Elmiron lawsuit.
On October 20, 2021, Oregon resident Deborah Quick filed a lawsuit against Janssen Pharmaceuticals alleging that her prolonged use of Elmiron caused macular eye damage. Quick initially filed her Complaint against Janssen in federal court in Oregon and it was subsequently transferred into the Elmiron MDL.
Quick was prescribed Elmiron in 2002 to help treat a chronic bladder condition (interstitial cystitis). She took Elmiron daily, as prescribed by her doctor, from 2002 until March 2020 without any interruption. After “numerous years of continuous use of Elmiron,” Quick began having serious problems with her vision. In December 2019, Quick was informed, for the first time, that her vision problems might be related to her use of Elmiron. Quick was soon diagnosed with Elmiron-induced pigmentary maculopathy.
In her Complaint, Quick alleges that Janssen had known for years about the risk of Elmiron use and macular eye damage but deliberately or negligently concealed that information. Quick is seeking damages against Janssen under seven separate causes of action including design defect, failure to warn, and general negligence.
How many Elmiron lawsuits have been filed?
There are over 1,700 Elmiron lawsuits pending in federal court at last count on November 15, 2022.
How does Elmiron damage the eyes?
Researchers have observed that eyes damaged by Elmiron use have over-pigmentation and yellow-orange deposits in the retinal pigment epithelium (RPE). What does that mean? The RPE is a layer of cells in the retina that has several functions, including light absorption. And, the over-pigmentation and deposits are a signal that something has gone wrong.
The mechanism by which the eyes are damaged by the drug is not entirely clear. There is an unknown reaction that occurs between Elmiron and cells in the RPE. This interaction may be because of a “matching-up” in the shapes of the Elmiron molecules and the RPE cells, rather like puzzle pieces.
Should I stop taking Elmiron?
Sadly, discontinuing Elmiron may not prevent eye damage from progressing. A case study published in 2019 of a patient who had stopped taking the drug continued to have eye degeneration for 6 years.
However, researchers have suggested that stopping the medication may mitigate eye damage. If the damage has progressed too far, though, it may not be preventable. Most importantly, people taking Elmiron should immediately see their doctors and get screened for retinal damage.
The tough part about stopping this medication is that it is one of the only medications used to treat the chronic, untreatable IC condition. I have a lawyer friend who has this very problem. There are other medications and treatments available. But Elmiron is a great drug for interstitial cystitis. This creates a Hobson’s choice for people who suffer from IC.
So what do you do? Discuss with your healthcare provider and weigh with her the risk and benefits. That is all you can do.
This advice also underscores the premise of these Elmiron eye damage lawsuits, right? Lawyers in the Elmiron class action lawsuit are not screaming for a recall. Instead, we are saying put a label on the product warning of the risk so doctors and patients can make the best possible choice.
Could I have been misdiagnosed with age-related macular degeneration?
This new type of maculopathy, caused by chronic exposure to Elmiron, was just discovered in 2018. This means that you may have been diagnosed with another condition incorrectly. You may still face that problem if you go to the doctor today because of a lack of awareness about the condition. If you go to the doctor to get your eyes checked, be sure to mention that you take or have taken Elmiron.
PPM may be misdiagnosed as age-related macular degeneration, pattern dystrophy, or another condition.
Who Can File an Elmiron Pigmentary Maculopathy Lawsuit?
Our lawyers are looking another who has a strong history of Elmiron use and an eye injury that may be related to the drug.
How do I file an Elmiron lawsuit?
Typically, cases like these involving hundreds of plaintiffs are not handled one by one. Instead, they are typically consolidated under one federal judge. You will file your case in your home state, it will probably end up in multidistrict litigation (MDL). We expect all cases to soon be consolidated under one federal court judge. Where? We have no idea at this point.
After lawyers gather information during a discovery period, the judge may hold “bellwether” trials. These are example trials that are used to gauge how much the cases are worth. Most claims will be settled after these trials.
How much are Elmiron settlements?
There have not been Elmiron settlements in the MDL class action lawsuit. So far, Janssen Pharmaceuticals and other defendants have held firm.
But our Elmiron lawyers are very bullish on these cases and believe many of these lawsuits will have a very high settlement value compared to other class action mass tort cases. The average Elmiron settlement amount is likely to be north of $250,000 and the average Elmiron verdict will likely be in the millions.
So we expect Elmiron compensation will eventually compensate victims for what they have endured.
Are there Elmiron lawsuits in state court?
A growing number of Elmiron lawsuits have been getting filed in the state courts for New Jersey, which is where Janssen is headquartered.
On September 9, 2021, a petition was filed with the New Jersey Supreme Court requesting that all Elmiron cases pending in NJ be consolidated into a Multicounty Litigation (“MCL”).
The MCL is New Jersey’s state court version of an MDL. Currently, there appear to be around 30 Elmiron cases in New Jersey state courts, with 20 pending in Mercer County.
A decision on the MCL request will be made in October.
Contact an Elmiron lawyer
Filing a lawsuit against a pharmaceutical company can be daunting. The Elmiron trials are still in their infancy, and we do not yet know what they will be worth. However, we believe that there is money available to you to make up for the injuries you have suffered. At Miller & Zois, we are experienced in drug mass actions. Call us at (800) 553-8082 or go online for a free consultation.
Bibliography
- Association of macular disease with long-term use of pentosan polysulfate sodium: findings from a US cohort by Neiraj Jain et al., British Journal of Ophthalmology, November 2019.
A study that used a database to look at thousands of people who took PPS. Researchers searched to see if any had a new diagnosis of macular disease. They found that 7 years later, PPS was associated with macular disease.
- Chronic Exposure to Pentosan Polysulfate Sodium is Associated with Retinal Pigmentary Changes and Vision Loss by Janelle Foote et al., The Journal of Urology, April 2019.
This study outlines the physical changes observed in the eye when taking PPS. The authors suggest discontinuing the use of PPS.
- A case of pentosan polysulfate maculopathy originally diagnosed as Stargardt disease by Robin Vora et al., American Journal of Ophthalmology Case Reports, March 2020.
In this case study, the authors describe a patient whose maculopathy associated with taking PPS was diagnosed as a different condition. This case study highlights the importance of looking at a patient’s history and catching drug toxicity before it causes further damage.
- Possible drug-induced, vision-threatening maculopathy secondary to chronic pentosan polysulfate sodium (Elmiron®) exposure by R. Christopher Doiran et al., Canadian Urological Association Journal, January 2020.
A summary of the most important studies brought this issue to light.
- Clinical Pearls for A New Condition by Adam Hanif and Neiraj Jain, Review of Ophthalmology, July 2019.
This article, written by the leading scientists who discovered this condition, provides an overview of the science behind PPS and maculopathy, including who is affected. Chronic use, the article clarifies, is needed for eyes to be damaged.
 Maryland Injury Law Center
Maryland Injury Law Center